Porter’s Papers – A review of recent literature from Rod Porter
(Full text of Rod’s bi-monthly newsletters, which include med chem literature reviews in addition to the more general/comp chem papers included here, can be found on his website at http://rodporterconsultancy.com/newsletter/).
Virtual ADMET modelling
A couple of papers discuss the calculated and physicochemical properties relating to promiscuity. First up1 is a paper looking at 3D descriptors and promiscuity as measured by successful clinical progression of compounds. The authors suggest that shape-based 3D descriptors such as the radius of gyration and shadow indices discriminate off-target promiscuity of a set nicotinic ligands better than do fraction of sp3 carbon (Fsp3) and the number of stereo centres which others2 have proposed as key indicators of clinical success. The authors also extended this analysis to show these values were predictors of progress through preclinical and Ph1 development and were also found to be good indicators of solubility. Shadow Index or Fsp3 critieria were generally met (84%) by marketed drugs but were not met by withdrawn or discontinued products. It appears that spherical compounds with few aromatic rings have a better chance of making drugs – does this help explain some of the upsides of macrocycles? I must admit I am not quite convinced about the robust of the arguments over selectivity here.
s a bit of a contrast Keseru3emphasises the importance of LogP and basicity in determining selectivity which rather reinforces my feelings against the routine introduction of basic centres to make a compound soluble – there are other ways of doing this – see above – apart from anything else.
A warning comes from Kenny and Montanari4 that one does have to be careful with data analysis as some strategies can exaggerate the significance of relationships –they introduce a term “correlation inflation”. They particularly highlight the risk of analysing binned data and averaging groups of data points before analysis
With respect to promiscuity there is a lot to be said for some targeted screening to look for particular nasties such as hERG or 5-HT2B receptor affinity an approach extolled by a group of authors5 sharing in vitro screening data from four major pharma.
Following the in silico or physicochemical parameters and attrition theme is a paper from Wenlock6 reviewing the relevance to ADMET behaviour of a range of physicochemical properties including, perhaps what may be considered the usual suspects of, ionization lipophilicity, H-bonding and solubility amongst others – both measured and predicted.
- D. C. Kombo et al, J. Chem. Inf. Model., Article ASAP DOI: 10.1021/ci300445e Publication Date (Web): January 18, 2013
- F. Lovering et al J. Med. Chem., 2009, 52, 6752
- A Tarcsay and G. M. Keserű J. Med. Chem., Article ASAP DOI: 10.1021/jm301514n Publication Date (Web): January 28, 2013
- P. W. Kenny, C. A. Montanari J. of Computer-Aided Mol. Design Published online 10 January 2013
- J. Bowes et al, Nature Reviews Drug Discovery, 2012, 11, 909-922 doi:10.1038/nrd3845
- M. C. Wenlock and P. Barton Mol. Pharmaceutics, Article ASAP DOI: 10.1021/mp300537k Publication Date (Web): January 24, 2013
Predicting PK & Safety
A valuable review of the application of in silico, in vitro and in vivo PK for the prediction of human PK from AZ is just out1. It appears to be targeted to DMPK scientists but it seems pretty handy as a survey of the field to me as a medicinal chemist.
Another hazard for screening is concern over metal impurities in samples. An article from Roche2 discusses the problem of zinc contamination that they have had problems with. The recommended solution by the authors is to run a counter screen in the presence of metal chelator PTEN.
1. K. H. Grime, P. Barton and D. F. McGinnity Mol. Pharmaceutics, Article ASAP DOI: 10.1021/mp300476z Publication Date (Web): January 29, 2013
2. J. C. Hermann, et al ACS Med. Chem. Lett., Article ASAP, DOI:10.1021/ml3003296 Publication Date (Web): December 20, 2012
Halogen bonding
A comprehensive review on halogen bonding in medicinal chemistry makes a convincing case that we should forget regarding halogens as simply lipophilic blobs. The key point is the recognition of the anisotropic distribution of electrons in ArHal – excepting fluorine with its extreme electronegativity – in which a positively charged surface, the sigma hole, on the z-axis is available for interaction with a Lewis base. Covered in the review are a consideration of the strength of interactions and their manipulation by additional electron withdrawing substituents on an aromatic ring; interaction geometries and energy boundaries with a comparison of theory and practise – based on crystallography studies; a survey of successful application of halogen bonding and finally a defense of halogen. Halogens have often been regarded as a liability – too lipophilic and adding a lot of molecular weight for very little specific gain in target interaction – “its just a lipophilic effect”. The authors argue that affinity gains can be substantial claiming upto 100 fold for ArH – ArI based on an overt halogen bond and that while they are dense their actual contribution to molecular size is much below that of their molecular weight
R Wilcken et al J. Med. Chem., Article ASAP Publication Date (Web): January 03, 2013
Peptides = drugs?
Peptides as drugs can be an emotive subject for drug discovery scientists. A recent review1 highlights recent findings from a variety of fields that are converging on a new understanding of how conformation controls peptide bioactivity and bioavailability. The authors review some examples of peptides with significant oral bioactivity including, amongst others, α-amanitin a relatively rigid bicyclic peptide and the ubiquitous, more flexible, monocycle cyclosporine. Reviewing the case histories the authors conclude that cyclization is the key first step the trick of course being to find the relevant conformation. Refining the structure then follows with natural and unnatural amino macid substitutions and alkylations to optimise target affinity and physcicochemcial properties. However rational approachesa at this stage are limited. One thing that struck me was that often at least bicyclic structures are required – a bit more of a challenge synthetically.
A disappointment is that the authors do quote data on cell permeability in some instances with dye labelled analogues of a peptide and propose this is evidence of permeability of the peptide.
One of the big hopes for peptide drug discovery has been stapled peptides as alpha helix mimetics2. However recent data3 looking at stapled stabilized BimBH3 peptides suggest that this strategy is far from a universal panacea with loss of both target and cell activity on stapling acyclic peptides.
Snake/conotoxin peptides have been recognised for a long time as having some spectacular biological activities therefore a paper on mambalgins4, analgesic three finger acid sensing ion channels inhibitors isolated from Black Mamba toxin, is timely. Note these mambalgins are highly constrained with four disulfide bridges.
1. J. E. Bock et al ACS Chem. Biol. 2013 DOI: 10.1021/cb300515u
2.G. L. Verdine and G. J. Hilinski Methods Enzymol. 2012;503:3-33. doi: 10.1016/B978-0-12-396962-0.00001-X.
3. T Okamoto et al ACS Chem Biol., Article ASAP DOI: 10.1021/cb3005403 Publication Date (Web): November 14, 2012
4. S. Diochot et al Nature 2012, 490, 552 and A Fleming Nature Reviews Drug Discovery 2012, 11, 906
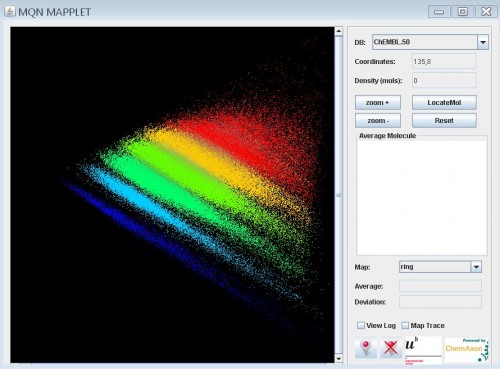
Interactive chemical space visualisation
A mapplet for the interactive visualisation of chemical space defined by principal component planes of 42 dimensional Molecular Quantum Numbers has just been introduced. Databases used for this purpoose are Drugbank, GDB-13 (near 1 billion molecules), GDB-11, Pubchem and Chembl. The graphic below illustrates the whole Chembl database, any molecule can be identified along with nearest neighbours. Zooming in allows selection of each molecule (not in GDB-13) otherwise each pixel may represent multiple molecules for which an average structure is displayed in the average molecule box. More information is available on the Reymond group website and in the reference.
1. M. Awale et al J. Chem. Inf. Model., Article ASAP DOI: 10.1021/ci300513m Publication Date (Web): January 22, 2013 Copyright © 2013